
How does Belviq (or Lorcaserin) Work? Is Belviq safe?

Return to KirkLindstrom.com home page

|
Arena's Belviq Approved by FDA for Obesity How does Belviq (or Lorcaserin) Work? Is Belviq safe? |

|
| Return to KirkLindstrom.com home page |
|
|
| June 27, 2012: Today the
U.S. Food and Drug Administration (FDA) today approved Belviq
(lorcaserin hydrochloride), as an addition to a reduced-calorie diet and
exercise, for chronic weight management. The FDA approved Belviq for
use in adults with a body mass index (BMI) of 30 or greater (obese), or
adults with a BMI of 27 or greater (overweight) and who have at least
one weight-related condition such as high blood pressure (hypertension),
type 2 diabetes, or high cholesterol (dyslipidemia). Belviq is Arena Pharmaceuticals, Inc. (Ticker ARNA) new weight-loss drug. The stock soared on the news. 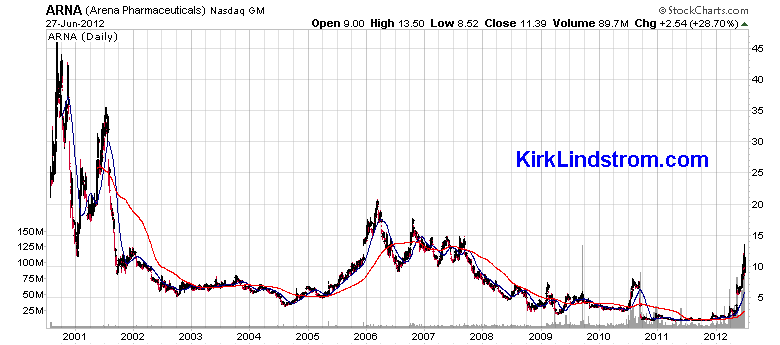 How does Belviq work? The FDA says1: Belviq (lorcaserin hydrochloride works by activating the serotonin 2C receptor in the brain. Activation of this receptor may help a person eat less and feel full after eating smaller amounts of food. |
|
|
How effective is Belviq? The FDA says:
The safety and efficacy of Belviq were evaluated in three randomized, placebo-controlled trials that included nearly 8,000 obese and overweight patients, with and without type 2 diabetes, treated for 52 to 104 weeks. All participants received lifestyle modification that consisted of a reduced calorie diet and exercise counseling. Compared with placebo, treatment with Belviq for up to one year was associated with average weight loss ranging from 3 percent to 3.7 percent.Is Belviq safe? The FDA says: Belviq should not be used during pregnancy. Treatment with Belviq may cause serious side effects, including serotonin syndrome, particularly when taken with certain medicines that increase serotonin levels or activate serotonin receptors. These include, but are not limited to, drugs commonly used to treat depression and migraine. Belviq may also cause disturbances in attention or memory.....
|
|
|
Article: Beware
of
Annuities
Article
Index
|
|
 |
|
| For quotes rather than graphs, click US Treasury Rate Quotes | |||
 |
KirkLindstrom.com Home of "CORE & Explore®" investing. |
Blog |
|
Disclaimer: The information contained in this seb site is not intended to constitute financial advice, and is not a recommendation or solicitation to buy, sell or hold any security. This blog is strictly informational and educational and is not to be construed as any kind of financial advice, investment advice or legal advice. Copyright © 2012 Kirk Lindstrom. Note: "CORE & Explore®" was coined by and is a registered trademark of Charles Schwab & Co., Inc. |
|||